In 2005, Active Motif acquired Chromeon GmbH, a German company established in 2001 by Professor Otto Wolfbeis, a world leader in fluorescent biosensors. Chromeon GmbH had identified early on the need to develop improved fluorescence-based biological assays.
By pairing the assay development of Active Motif with the fluorescent chemistry of Chromeon, we strive to develop innovative cell and molecular biology assays that incorporate the proprietary fluorescent dyes and substrates developed at Chromeon GmbH. Providing sensitivity and flexibility, Active Motif Chromeo dyes exhibit superior luminescence properties, including a broad range of fluorescence excitation and emission, large Stokes shifts, limited photobleaching and a broad pH tolerance.
Fluorescent Microscopy with fluorescently labeled antibodies (Immunofluorescence, or IF) is widely used to detect specific proteins in their cellular environment, and to answer questions regarding their localization, modification, interactions and life cycle. However, until recently the use of fluorescence microscopy in cell biology research has been limited by the resolution that can be achieved in imaging experiments.
The resolution of images is limited to the size of the spot that the excitation beam of the microscope can be focused. This is dependent on the wavelength of the emitting light and the parameters of the instrument. The highest resolution achievable is defined by Abbe's Law:
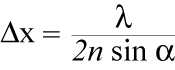
Figure 1: The Abbe Law describes the resolution that is achievable in microscopy.x = spatial resolution lambda = wavelength of the excited light
2n sin α = numerical aperture of the microscope
The Abbe limit restricts the ability of the observer to visually resolve objects separated by less than ~200 nm. This makes it impossible to study smaller bacteria, viruses, ribosomes or protein clusters by fluorescence microscopy. However, within the last few years several novel technologies have been developed that overcome this limitation.
Super-resolution fluorescence microscopy (also known as high-resolution microscopy) describes a group of techniques that “break” the diffraction barrier, enabling the resolution to be as low as 20-30 nm. These techniques take advantage of special physics & instrument settings, and the properties of high-quality fluorescent dyes to improve resolution dramatically. Although these techniques can be separated into two different approaches, they have one common basis: the ability to turn fluorescent molecules off when desired.
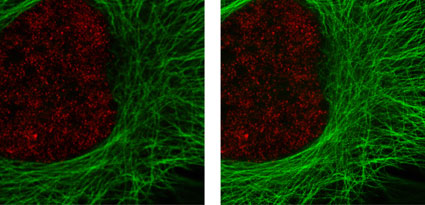
Figure 2: Active Motif's primary antibodies and fluorescent secondary antibodies in confocal and STED microscopy.
HeLa cells were stained with alpha Tubulin mouse mAb (Clone 5-B-1-2) and ATTO 647N (STED/GSD) Goat anti-mouse IgG (Catalog No. 15038). Histone H3 was stained with Histone H3 trimethyl Lys4 rabbit polyclonal antibody (Catalog No. 39159) and the Chromeo 488 Goat anti-rabbit IgG (Catalog No. 15041) secondary antibody. The confocal (left) and STED (right) images are courtesy of Leica Microsystems, Germany.
Super-resolution by direct optical imaging
The first approach is based on confocal microscopy, where molecules in the outer regions of the focal spot are turned off. This reduces the size of the fluorescent spot, as only dyes in the center of the excitation area are allowed to emit fluorescence. STED (STimulated Emission Depletion) and STED CW (Continuous Wave) belong to these direct optical imaging techniques. They do not require any computational evaluation as the samples are scanned in the normal manner. For more information please visit our STED microscopy products pages.
Super-resolution by localization
In this approach the majority of the molecules within the excited area are turned into a dark state; only some single dye-molecules are allowed to emit fluorescent light. The position of these individual molecules is determined by a mathematical algorithm, and each is visualized. By repeating the cycle of turning dyes off and detecting the fluorescence of the remaining individual emitters, thousands of images are created; these images are combined to form the final high-resolution image. Several techniques belong to this class of stochastic readout of individual molecule localization: STORM (STochastic Optical Reconstruction Microscopy), PALM (PhotoActivated Localization Microscopy) and GSDIM (Ground State Depletion with Individual Molecule return). For more information on localization based microscopy, please visit our GSDIM microscopy products pages.
With high-resolution microscopy it is possible to exceed the Abbe limit and achieve resolution improvements of up to 12-fold over classical confocal microscopy. The ability to perform imaging experiments at resolutions below 50 nm facilitates the separation of sub-cellular structures that previously could not be resolved, and increases the confidence in the biological roles of proteins and structures that co-localize.

